Hi guys BrandnB is here to share some info on amino-acids. Hope you pay attention.
So We know that Amino-acids are generally important but do you know the formula. In class, I was like it has nitrogen, hydrogen, carbon and oxygen. These are some of the important atoms that exist in our biochemical reactions.
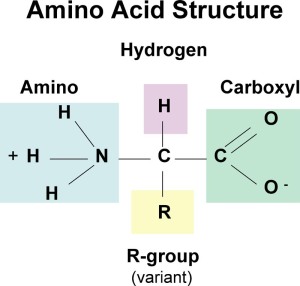
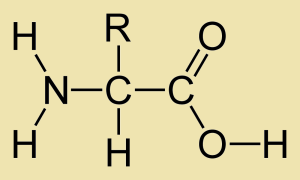
Amino-acids have a general structure that consists of an amino group, alpha carbon, carboxyl group and R’ chain( hydrocarbon attachment).
The amino-acids exist as a charged species in ionic form or neutral species ( non-ionic). This depends on ?????, the charges present on the terminal ends of course. Compare these forms, the one above and the one below. Which is the ionic species?
If you chose the first one then you won a prize of………..knowing the difference of a charge.
Wait there’s more…..
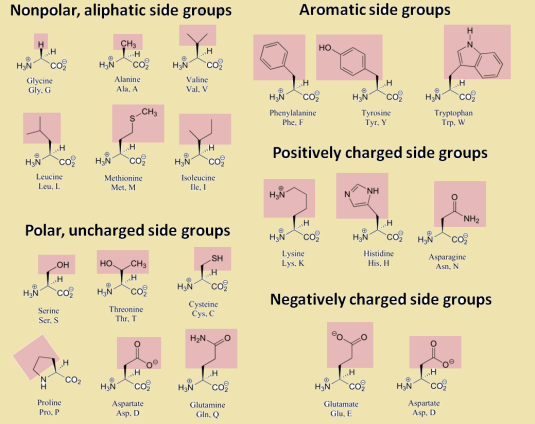
Amino acids have certain categories that exist in terms of its structure and their interacts with water and even themselves.
Amino-acids are unique in their structure but there exists various forms like polar uncharged R groups, non-polar groups, positively charged, negatively charged and aromatic.
Polarity and non-polarity are important in amino-acid structure and reactivity with each other. Polar groups can interact with each other to form connective structures that leads the formation of hydrogen bonds with the amide group and carbonyl group.
More importantly amino-acids bond together to create peptide bonds or petide linkages to create a chain of amino-acids. That reaction is fueled by a condesation reaction. Note the positioning of the amino group and the carbonyl group.
Indeed Patrick, this helps in adding amino-acids to the peptide chain as more amino acids add to it. The carboxyl group must be exposed in order to react with the amino end of the adjacent amino-acid.
It’s a condensation reaction due to the fact water was lost from both structures combining. The important linkage is the bond of the carbonyl carbon of the carboxyl group to the nitrogen on the amino group. Hence the link is quite mutual in forming a polypetide chain.
Since on the topic of bond formation, other bonds do exist in the bonding of amino-acids. The disulphide bridge/linkage between two amino-acids call cysteine. The oxidation of two molecules allows for cystine to be formed and ” bridge” the gap. The reaction is reversible by reduction of the cystine to two individual cysteine molecules. This type of bonding is present in the structures of tertiary proteins in which we will see in oncoming posts.
A dipetide is a chain formed between two amino acids and polypetide is between two or twenty amino acids long. Upon mentioning the number twenty there exists 20 esstential amino acids that you must obtain through dietary means.
To answer your question and that strange figure above amino acids can be obtained through everyday diet. Meats and eggs are primary sources of amino-acids which are building blocks of proteins. For some of us that are a little reluctant of consuming meat, beans and peas are alternative sources of amino acids. Plants which are our meatless……fat less…and minimal amino providing selection allow for creatine deficiency in our muscles. Creatine is an amino-acid that is a storage molecule for energy in our muscles and brains. Ever wonder about plants versus zombies, zombies eats plants to get to what? the BRAINS of course. For that much needed creatine to keep those dead bodies moving.
Bear Grylls understands too much about proteins in his daring adventures, we know how he washes all of that down….. Since he resides in nature for all of us to experience the wild to our comfort with our cable television, air conditioning, lighting, bathrooms, internet and other household essentials he knows the natural proteins are the best.
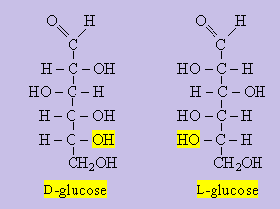
Wait a hint has appeared remember for glucose that the D isomer is the most useful and exists commonly compared to the L isomer.
L isomers of amino acids are the common form compared to the D isomer. So the more natural the better. Won’t you say Bear….
Back to the Isomerism of the amino acids.
The identification of the L isomer is determined by the placement of the atoms around the alpha carbon. The structures are arranged into the Fischer projections. The carbon molecules align themselves and the amino group assumes on the left or right of the chiral carbon. If the group is found on the left of the asymmetric carbon it is named L-isomer. So D isomer exists as the right…..
Functions Of Proteins.
They can acts as biological catalysts, storage molecules( ferritin), transport molecules of Oxygen and Carbon dioxide (hemoglobin), Protein pumps and protein channels and they assist in muscle growth. Due to different functional roles of proteins their structure differ from task to task.
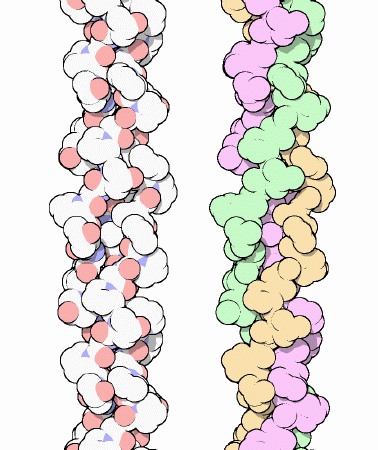
There exist the primary(1) structure: that is in a linear arrangement and the amino-acids can be identified in a particular order due the peptide bonds through out.
The secondary(2) structure consists of habitual folding patterns or an individual strain being warped around. They are the beta pleated sheet and the alpha helix. the beat pleated sheet can be represented by a pleated skirt or something like that and the alpha helix is like a telephone cord if got one that curls, just so you know cordless phones have none. The alpha helix contort to adjust themselves every 4th amino acid to create a hydrogen bond of the electronegative oxygen on the carbonyl group with the hydrogen of amino group. These structures in alpha helices can be disrupted by large hydrocarbon chains and other amino-acids.
The tertiary ( 3) structure of proteins are a little more complex with more bonding attributes like hydrogen bonding, salt bridges and disulphide bridges. This is essential for the tertiary structures because various combinations allow for variety.
Structural proteins like Collagen: Note the triple helix.
Example myoglobin for oxygen storage:
The Quaternary structure is a much more complex arrangement. In these structures subunits can combine to form structures like haemoglobin with side chains and iron groups:
As I come to a close I can share some interesting things I learnt in my lecture about Arginine, this amino-acid is responsible for the expansion of blood vessels. So it can assist for heart defects and help in blood pressure regulation. Gym buffs can utilize this for max reps.!!! .
 So certain amino-acids can be determined by the Ninhydrin test which produces a purple color.
So certain amino-acids can be determined by the Ninhydrin test which produces a purple color.
Proteins can be determine by the biuret test that involves the reduction of copper(II) ions due to linking with the nitrogen. The basic solution of copper sulphate allows the hydrogen attached to the nitrogen to be removed.
In closing I leave this with you,
Elkaradagi S,Protein Structure and Structural Bioinformatics, http://www.proteinstructures.com/Structure/Structure/amino-acids.html(Feb,16,2014).
S. F. Betz,Disulfide bonds and the stability of globular proteins.,http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2142256/(Feb,16,2014)