Hey guys, Kimberly and Aaliyah here this week and we’re talking nucleotides and nucleic acids!
So we’ve learned before about the “central dogma” of molecular biology that basically encompasses the transcription from DNA to RNA and then the translation from RNA to protein.

Did you ever stop to think about what these things might be made of, however? Well today you’re gonna get the long-awaited answer for your question 😛
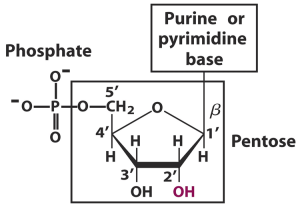
DNA and RNA are made up of nucleotides. These are the basic units or the building blocks of nucleic acids. Nucleotides are made up of three components- a sugar, a base and a phosphate group.
Components of Nucleotides:
Sugar: The first component we will talk about is the sugar part of a nucleotide. This is a 5-carbon sugar otherwise known as a pentose. Two kinds of these sugars are found in nucleotides. These are deoxyribose, which has a single hydrogen atom (H) attached to its carbon 2, and ribose, which has a hydroxyl group (-OH) attached to the carbon 2 instead. Deoxyribose is found in DNA while ribose is found in RNA. Deoxyribose gets its name simply because it is ribose that has lost an oxygen atom.

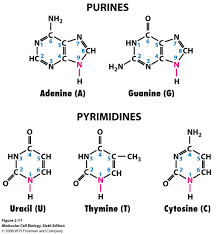
Base: The bases used in making up nucleotides are heterocyclic bases or nitrogenous bases (because they have a nitrogen incorporated into the ring, duh!). These bases can be separated into two types- the pyramidines and the purines. These have one ring, and are cytosine (C), thymine (T) and uracil (U), and two rings, and are adenine (A) and guanine (G) respectively. The pyramidines in DNA are cytosine and thymine and the pyramidines in RNA are cytosine and uracil, while the two purines, adanine and guanine are found in both DNA and RNA.
Phosphate: For nucleotide polymerization to occur and for these nucleic acids to be formed, a phosphate group must be present.
Altogether, these groups make up one nucleotide illustrated in the picture below.

When a pentose sugar is bonded to the base alone, the structure is called a nucleoside. When phosphate group is added to the nucleoside, it becomes a nucletotide 🙂
DNAis made up of two polynucleotide chains, which are chains that are made by the joining together of nucleotide monomers that coil around each other. They not only coil around each other, but have bonds between the chains that hold the two polynucleotide chains together. This double helix is held together by hydrogen bonds that are formed between the bases of both chains. This occurs via complementary base pairing where adenine forms two hydrogen bonds with thymine and cytosine forms three hydrogen bonds with guanine.

It’s pretty simple once you see it in a picture isn’t it? Kinda looks like a twisted ladder, right? Lol Just think of the ladder rungs being the amine bases all paired up joined by hydrogen bonding, and the backbones being the sugar and phosphate parts! Easy like a Sunday morning.
Now where do bases attach to nucleic acids?
- C1 position of ribose or deoxyribose
- Pyrimidines attach via their N1 position of the ring to the pentose
- Purines attach through the N9 position
- Other bases(minor) have different attachments.
ROLES OF NUCLEOTIDES
- Building material of nucleic acids (RNA,DNA)
- Provide energy in cellular metabolism (energy currency- ATP)
- Allosteric effectors
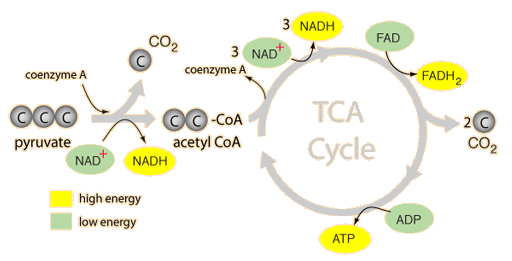
- Enzyme cofactors are structurally made up of nucleotides eg NAD
ROLES OF NUCLEIC ACIDS
1) genes – information needed for functional protein and RNA synthesis
2) promotors- segments which are involved in gene expression regulators
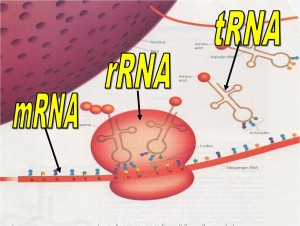
- rRNAs (ribosome RNA) – made up of ribosomes which are involved in synthesis of proteins.
- mRNAs (messenger RNA) – acts as a messenger owl carrying genetic information from gene to ribosome.
- tRNA (transfer RNA) – information taken from mRNA is then translated into an amino acid sequence.
- In some cases, RNAs perform catalysis.
Huh guys, imagine life without nucleotides or nucleic acids, do you think we would have even existed! So I guess you can’t imagine life cause there would be none!
ATP
- Adenosine triphosphate (nucleotide)
- Contains adenine, ribose and triphosphate group
- Energy carrier
- Provides energy for other cells which perform jobs such as biosynthetic reactions, ion transport and cell movement.
- Energy of ATP is made when the transfer of 1 or 2 of its phosphate groups to another molecule occurs. ADP + Pi —–à ATP
- Reverse reaction is the hydrolysis of ATP to ADP.

http://hyperphysics.phy-astr.gsu.edu/hbase/biology/atp.html
NAD


NUCLEOTIDE REGULATION
- COVALENT MODFICATION– activates some enzymes and inactivates others because enzymes are inactive until they are covalently modified so they could work. Eg Phosphorylation
NUCLEIC ACIDS
– Polynucleotides
– Oxygen at 5’ end of one nucleotide is connected to the oxygen of the 3’ end of another.
– 
NUCLEIC ACID STRUCTURE
– The sugar phosphate is the backbone of the nucleotide
– There are 2 separate strands:
1) Parallel (3’à5’ direction)
2) Antiparallel (5’à3’ direction)
– Base pairing – hydrogen bonding holding the 2 strands together.
– 
– http://www.visionlearning.com/en/library/Biology/2/DNA-II/160
This structure is important in:
– DNA replication
– RNA transcription

– Backbones are the sugar –phosphate which are negatively charged so it is located outside
– Planner bases are stacked above each other like pancakes however on the inside
HOW ARE NUCLEIC ACIDS FORMED?
– Monomers of nucleotides are joined by phosphodiester linkage which is a formation between the hydroxyl (OH) group at the 3’ end of nucleotide and phosphate of a next nucleotide.
– The 5’end then lacks a nucleotide at the 5’ position and the 3’ end lacks a nucleotide at the 3’ position.
HELICAL TURN
– 10 base pairs per turn
– 34 amino acids per turn
– There are 3 helices called:
1) A form
2) B form
3) Z form

NUCLEIC ACIDS
– Most common form for DNA is the B form. It is the standard DNA double helix.
– Most common form for RNA is the A form. WHY?
– Because of its deeper minor groove and shallow major groove. It is also favoured in low water conditions.
– Z form:
– Has a narrow, deep minor groove
– Has no major groove
– Can form some DNA
– However requires alternating syn and anti base configurations
– It is a laboratory anomaly
– Because of its high salt or charge neutralization and it is left handed while most helices are right handed.

B- DNA
– Has a deeper major groove and a
– Shallow minor groove
RNA
– rRNA – RNA + PROTEIN -à RIBOSOMES
– mRNA
o carries DNA code to cytosol for protein synthesis or ribosomes.
o Are read in codons or triplets from (5’ to 3’ direction)
– tRNA
o carries amino acids to mRNA and like a puzzle matches up by base-pairing of anti codon with mRNA codon.

STABILITY OF NUCLEIC ACIDS
There are many factors which stabilize and destabilize the helix of nucleic acids. They are:
1) HYDROGEN BONDING
o Contributes to DNA double helix, RNA secondary structure
2) STACKING INTERACTION OR HYDROPHOBIC INTERACTION BETWEEN BASE PAIRS
o It is energetically favourable
o Maximized stacking in double-stranded DNA
EFFECT OF ACID ON NUCLEIC ACIDS
– Strong acid and high temperatures
o They are hydrolysed to base,riboses or deoxyriboses and phosphate.
– At pH 3-4 apurinic nucleic acids are formed. This is due to the glycosylic bonds attaching the purine A and G bases to the ribose ring are broken.
– However formic acid regenerates this.
EFFECT OF ALKALI ON NUCLEIC ACIDS
– HIGH pH (>7-8)
o Small effects on DNA structure
– HIGH pH
o Changes the tautomeric (keto and enol forms) state of bases.
– Change in tautomeric states of bases results in DNA denaturation therefore it results in unstable base pairing.

– RNA is unstable at higher pH because of its 2 OH groups.
CHEMICAL DENATURATION OF NUCLEIC ACIDS
Two chemical which denature nucleic acids are:
-Urea (H2NCONH2)
– Formamide (HCONH2)
HOW IT OCCURS?
-Disrupts hydrogen bonding of water solution
-Reduction of hydrophobic effect between bases
-Denaturation of strands in double helix
BUOYANT DENSITY OF DNA
– 1.7gcm-3 = 8M CsCl
– It is used in DNA purification
C3 SPECTROSCOPIC AND THERMAL PROPERTIES OF NUCLEIC ACIDS
1) UV SPECTROSCOPY
o Absorption of UV light due to bases
o Maximum wavelength absorption by both DNA and RNA (Max=260nm)
o Applications: detection, quantitation , assessment of purity (A260/ A280)
2) HYPOCHROMICITY
o Creation of hydrophobic environment by stacking of bases making them less accessible to UV absorption (dsDNA, ssDNA or RNA nucleotide)
o
3) QUANTITATION OF NUCLEIC ACIDS
o Extinction coefficient- 1mg/ml dsDNA has an A260 of 20 ssDNA and 25 RNA
o The values for ssDNA and RNA are approximate
o Values are the sum of absorbance of different bases (purines are more than pyrimidines)
o Absorbance values depend on the amount of secondary structures due to hypochromicity or stacking of bases.
4) PURITY OF DNA
o A260/ A280
o DsDNA – 1.8
o Pure RNA -2.0
o Protein – 0.5
5) THERMAL DENATURATION/MELTING
o Destruction of the double stranded hydrogen bonded regions of DNA and RNA
o RNA – absorbance increases gradually and irregularly
o DNA- increases cooperatively.
o Tm- melting temperature. This the temperature at which a 40% increase in absorbance is achieved

6) RENATURATION
o Rapid cooling- only allows formation of the local base pairs and absorbance is slightly lowered.
o Slow cooling- the formation of the whole complementation of dsDNA and absorbance decreases gradually and cooperatively just like denaturation
OK guys i think that’s enough for nucleotides and nucleic acids. SEE YOU NEXT TIME! have a great weekend!